Gilson의 'Purification of Vanillin by a Combination of Flash and Preparative HPLC with the PLC 2250'을 이용한 응용자료는 한국분석기기(주)에서 제공하였으며 주요 내용은 다음과 같다.
INTRODUCTION
Many phenolic compounds found in plants have been shown to exhibit analgesic, antimicrobial, anti-inflammatory, antioxidant, antipyretic, and immuno-modulatory properties.
As scavengers of reactive oxygen species, these compounds show potential as therapeutic drugs in the treatment and management of cardiovascular diseases, neurodegenerative diseases, and cancer. Rapid and simple isolation of these bioactive compounds present in natural products is critical to the advancement of new medical treatments and therapies.1
''The Gilson PLC 2250 provides an automated single platform solution for bulk flash purification and refined preparative HPLC."
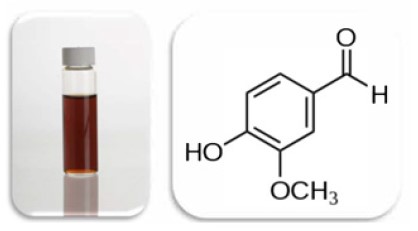
Vanillin, known best as the principal flavor component of vanilla extract(Figure 2), has also been shown to exhibit some of these bioactive properties.2
The manual isolation process can be time-consuming, difficult, and lead to inconsistent results.
The Gilson PLC 2250(Figure 1) provides an automated single platform solution for bulk flash purification and refined preparatory HPLC.
Figure 1. Gilson PLC 2O50.
Users can easily switch between flash and preparative chromatography with the turn of a lever.
Figure 2. (Left) Crude vanilla extract. (Right) structure of vanillin.
MATERIALS & METHODS
Vanillin was purified from commercial vanilla extract using flash chromatography followed by preparative HPLC(Gilson PLC 2250). Final yield and relative purity were determined by UV absorbance and TLC, respectively.
Sample and Standard Solutions
The sample, All-Natural Vanilla Extract(McCormick), was concentrated 10 fold before use by evaporation under nitrogen.
Standard vanillin solutions(0.1mg/mL to 1.0mg/mL) were prepared by dissolving powder reagent(99% Vanillin; Sigma-Aldrich ; V1104) in HPLC grade MeOH.
Instrumentation
Table 1. PLC2250 Configuration
Chromatography method
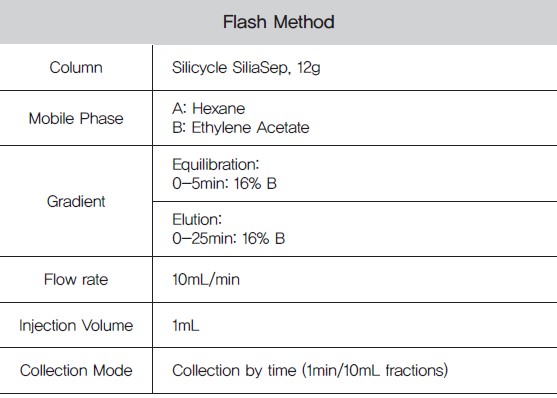
Table 2. Flash Method
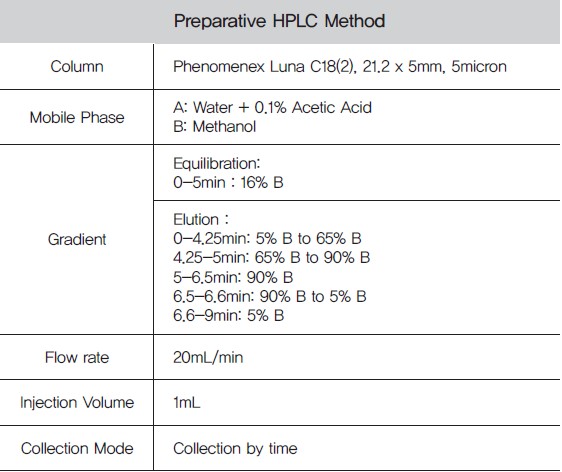
Table 3. Preparative HPLC Method
Analysis
Vanillin peak retention times were determined with 1mL vanillin standard injections(1mg/mL) using methods described in Tables 2 and 3. Final yield was calculated from the extinction coefficient of vanillin using a multi-mode plate reader(BMG CLARIOstar; absorbance at 347nm; εmM=25.1 for vanillin).
Relative purity was assessed by TLC(SiliaPlate™ TLC Plate, 50:50 Hexane: Ethyl Acetate).
RESULTS & DISCUSSION
Approximately 20mg of vanillin was isolated from 1mL of 10x concentrated vanilla extract through a combination of flash chromatography and preparative HPLC on the same instrument platform.
Normal phase flash chromatography using hexane: ethyl acetate as the mobile phase was successful in cleaning up the vanilla extract sample(Figure 3). The collected flash fractions containing vanillin(as determined by TLC, Figure 4) were then concentrated with nitrogen.
Figure 3. Flash purification of vanilla extract using hexane: ethyl acetate.
The mobile phase gradient is represented by a dashed horizontal red line(16% ethyl acetate, secondary axis). Vertical dashed lines indicate 3min intervals. Fractions(1min) are shown by numbers above the graph and corresponding colored bars near the bottom of the graph.
Vanillin peak eluted in fractions 18-20 between approximately 17 and 20min.
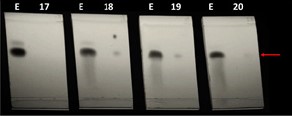
Figure 4. TLC was employed throughout the experiment to test chromatography conditions and to monitor the presence of vanillin in fractions. In this figure four fractions(fractions 17-20 from Figure 3) ware each separated on a TLC plate next to the vanilla extract sample(E).
Fraction 18, 19, and 20 contained vanillin(Red arrow) and were combined, evaporated, and subjected to preparative HPLC as shown in Figure 5.
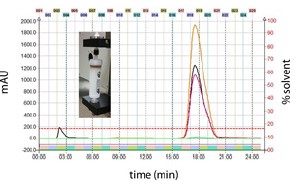
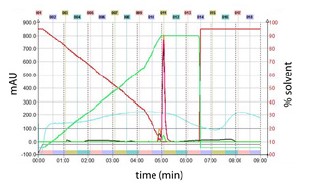
Figure 5. Prep HPLC was employed to purify vanillin from the collected flash fractions. The mobile phase gradient is represented by solid green(Methanol) and red(Water + 0.1% acetic acid) lines on the secondary axis.
Vertical dashed lines indicate 1min intervals. Fraction(30sec) are shown by numbers above the graph and corresponding colored bars near the bottom of the graph. Vanillin peak(280nm, primary axis) eluted in fractions 10 and 11at ~ 5min. Absorbance data are shown for 280nm(Orange), 312(Magenta), 347nm(Green) and scan(Blue).
With a simple valve switch on the PLC 2250, the concentrated flash fraction was then further purified on the preparative reverse phase C18 HPLC column(Figure 5) using a MeOH: acidified water gradient.3 The vanillin in the preparative HPLC fraction was of high purity as determined by TLC analysis(Data not shown).
Calculated of vanillin in the fraction was 1mg/mL. Taking into account the volume of the fraction and the concentration method used before flash purification, this corresponds to 2mg/mL in the original vanilla extract. This is near literature reported values for vanillin isolated from natural vanilla extract.4
Gilson Glider Prep software allows the user to quickly create flash and prep HPLC protocols for use on the Gilson PLC 2250.
Users can store column profiles for use in either method, quickly applying pressure limitations and flow rates to the method. The ability to monitor four wavelengths and perform absorption scans (Figure 6) enables easy observation of run-time peak purity.
The software also provides run reports for easy and complete record keeping.
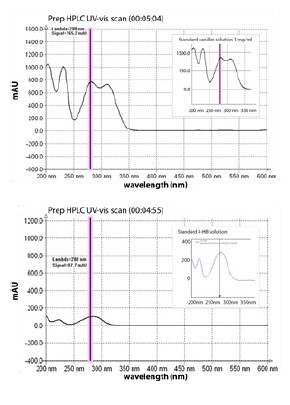
Figure 6. Top: Gilson Glider Prep screen shot of 200-600nm scan of vanillin peak at 5:04min(Fraction 11) during prep HPLC, with inset of UV-VIS spectrum of a 1mg/mL vanillin standard solution run under the same conditions.
Bottom: 200-600 nm scan of presumed 4-hydroxybenzaldehyde(4-HB) minor peak at 4:55(Fraction 10) during prep HPLC. inset show 4-HB standard.
The use of both normal phase flash and preparative reverse phase HPLC provides crude clean-up and more refined purification on the same instrument, saving time and money.
The initial flash chromatography step provided crude clean-up of the sample, removing impurities before subsequent injection on a preparative HPLC column.
The combination of flash and preparative HPLC enabled rapid purification of a greater sample volume that could be achieved by preparative HPLC alone, with the initial bulk flash purification protecting the prep HPLC column integrity upon subsequent injection. The four wavelength capability os the PLC 2250 detector provided thorough monitoring of the purification process.
SUMMARY
• Gilson Glider Prep software allows the user to quickly create flash and preparative HPLC methods, monitor run time conditions, and generate reports for easy and complete recordkeeping.
• Vanillin was isolated from all-natured vanilla extract using the Gilson PLC 2250, demonstrating the utility of the Gilson PLC 2250 in natural product purification with potential for product scale-up.
• The combination of flash and preparative chromatography available on the Gilson PLC 2250 enables bulk purification and refined Preparative HPLC on the same platform.
'Purification of Vanillin by a Combination of Flash and Preparative HPLC with the PLC 2250'에 대한 궁금한 내용은 본 원고자료를 제공한 한국분석기기(주)를 통하여 확인할 수 있다.
Reference(참고문헌): Application Note FB1015 (pp1-6)
1. Mradu, G., Saumyakanti, S., Cohini, M., Arup, M. HPLC Profiles of Standard Phenolic Compounds Present in Medicinal Plants.
Intl. Journal of Pharmacognosy and Phytochemical Research. 4, 162-167 (2012).
2. Kumar, R., Sharma, P.K., Mishra, P.S. A Review on the Vanilin Derivatives Showing Various Biological Activities.
International Journal of PharmTech Research. 4(1), 266-279 (2012).
3. Huesgen, A.G. Analysis of Natural and Artificial Vanilla Preparations, Agilent Technologies, Inc. Publication number 5990-8716EN. 1-11 (2011).
4. Hong, P., Jones, M., McConville, P. Authentication of Vanilla Extracts by Convergence Chromatography, Waters Corporation. Technical Note 720004701EN. 1-3 (2013).
Model Name(모델명): PLC 2250 System
The Person in Charge(담당자): Hyesook An
Maker(제조사): Gilson.
Country of Origin(원산지): France
Mail inquiry(기사문의): kaisco1@kaisco.co.kr
Data Services(자료제공): KAISCO.
<이 기사는 사이언스21 매거진 2021년 5월호에 게재 되었습니다.>