Gilson의 'EXTRACTMAN® : Rapid, Reproducible DNA Extraction with High Yield'를 이용한 응용자료는 한국분석기기(주)에서 제공하였으며 주요 내용은 다음과 같다.
"ABSTRACT
EXTRACTMAN® improved isolation speed nine-fold and produced equivalent or better yields of purified DNA compared to tube-based isolation methods. EXTRACTMAN® is versatile and compatible with a variety of existing paramagnetic bead-based isolation kits. Four DNA samples from two different organisms(mammalian and plant) were processed in parallel with no cross-contamination.
INTRODUCTION
DNA sequence analysis is an essential method in both research and diagnostics with applications in a wide range of scientific fields, including agricultural, biotechnology, food safety, forensics, and virology. In medical research and diagnostics, human DNA is evaluated for the presence of factors, such as the presence of viral DNA or mutations in cancer, which can affect the development and progression of disease. Effective and efficient sequence detection requires preparation methods that isolate and purify DNA from crude lysates and other complex biological samples.
“EXTRACTMAN® achieved four DNA isolations nine times faster than the tube-based method.”
In this application note, EXTRACTMAN®(Figure 1) was used to quickly, easily, and efficiently isolate DNA from crude preparations of mammalian and plant cells.
Figure 1. EXTRACTMAN® includes a base and handle and uses disposable microplates and bead capture strips.
The technology makes use of paramagnetic beads, also referred to as paramagnetic particles(PMP), with surfaces that bind specific analytes. Selective binding enables exclusion-based sample preparation(ESP™), a class of simple and fast purification techniques in which analyte bound to paramagnetic beads is pulled through a liquid interface into a second phase(air or liquid). Because ESP uses surface tension to gently and efficiently exclude unbound material from being transferred to the next phase, even weakly-bound species can be isolated.
Figure 2. EXTRACTMAN® is operated by repetition of two basic user movements after paramagnetic beads are first captured: 1) slide handle to release the paramagnetic beads and 2) slide release magnet to re-capture paramagnetic beads.
Release is achieved when the capture magnets in the handle and release magnets in the base are aligned over and under the same well column, repelling the capture magnets up into the handle. Re-capture is achieved by maintaining the position of the handle over the capture well and by advancing the release magnet to the next well column.
Figure 3. EXTRACTMAN® DNA isolation process using ESP™.
During the bind step, DNA in the crude lysate is selectively adsorbed to the paramagnetic beads. paramagnetic bead-bound DNA is then moved through wash buffer to the elution buffer using magnetic manipulation of the paramagnetic beads. Unbound contaminants remain in the input well and nonspecific carryover is eliminated via washes. Each step in the process requires only seconds of hands-on time.
EXTRACTMAN® employs novel techniques that enable ESP, offering a fundamentally new process of analyte purification(Figure 2).
With EXTRACTMAN®, paramagnetic beads bound to the analyte are uniquely manipulated through the bind, wash, elute workflow
using two opposing sets of magnets: (1) free-floating capture magnets housed in the sliding handle, and (2) manually actuated release magnets, which slide below the microplate and have opposing polarity to the capture magnets. Sample preparation proceeds in two steps. The first step involves paramagnetic bead capture onto the handle’s bead capture strip followed by sliding the handle to advance the bead capture strip(and associated paramagnetic beads) from a first column of wells to a second column.
The release magnet, initially positioned under the second column of wells, repels the capture magnets and attracts the paramagnetic beads from bead capture strip into the second column of wells. In the second step, the release magnets are advanced to a successive column of wells.
As a result, the capture magnets are no longer repelled by the release magnets, and fall back to the lower position to recapture the paramagnetic beads. Repeating these two steps across the microplate carries the paramagnetic beads bound to analyte from the input, through the washes, and to the output (Figure 3).
“…Gilson EXTRACTMAN® was used to quickly, easily, and efficiently isolate DNA…”
The process of capture-slide-release-slide allows the user to quickly perform four isolations in parallel. The simple sliding movements virtually eliminate user-to-user variability, whereas analyte recovery and purity in other existing manual techniques depend greatly on the user’s skill in the art of performing careful aspirations. Additionally, the simplicity of this ESP™ method has lent itself to straightforward automation on Gilson PIPETMAX®. Thus, the advantages of EXTRACTMAN® are easily scalable to higher throughput.
This application note describes DNA isolation from cell culture lysate by three commercial kits that were simply and easily adapted for use with EXTRACTMAN®.
EXTRACTMAN® achieved four DNA isolations nine times faster than the tube-based protocol (Figure 4). Translation of these commercial kits to EXTRACTMAN® resulted in improved or equivalent yield while maintaining sample purity and integrity.
Figure 4. Time required to complete four parallel DNA isolations using either EXTRACTMAN® or standard tube-based isolations. The EXTRACTMAN® method was on average approximately nine times faster than tube-based methods.
MATERIALS AND METHODS
Cell Culture
eGFP-expressing MCF7 breast cancer cells(a generous gift of Dr. Elaine Alarid, UW-Madison, Wisconsin) were maintained in a humidified chamber at 37℃ and 5% CO₂. Culture medium consisted of DMEM with 10% FBS and 1% penicillin-streptomycin. Cells were passaged and harvested using 0.25% trypsin-EDTA.
Kit Adaptation
Three kits were evaluated: Agencourt® DNAdvance™(Beckman Coulter; Brea, CA); Dynabeads® DNA DIRECT™ Universal Kit(Life Technologies; Carlsbad, CA); Magnesil® Paramagnetic Particles (Promega; Madison, WI) with Buffer RLT(QIAGEN; Germantown, MD). Tube‑based DNA isolations were performed with a 6-Tube Magnetic Separation Rack(New England BioLabs; Ipswich, MA). Kits were used according to manufacturers’ protocols with the following modifications: DNA was isolated from 104 cells for all kits, final volume for elution was 100μL for all kits, cell culture samples were used in place of tissue, lysis/digestion was shortened to 1 hour for AGENCOURT DNAdvance kit.
For EXTRACTMAN® isolations the DNAdvance and Dynabeads DNA DIRECT Universal Kit were further modified: sample lysate input volume was 420μL for all kits(maintaining 104 cells/isolation and paramagnetic bead number was the same as the tube-based equivalent), all wash and elution volumes were 100μL.
EXTRACTMAN® Device Operation
Paramagnetic bead-bound DNA was captured in the input and transferred to the wash wells by sliding the handle with the paramagnetic beads held in place on the bead capture strip by the capture magnets. The paramagnetic beads were released into the wash wells and re-captured by moving the release magnet under and away from the current wash well respectively. This wash sequence was repeated for the appropriate number of wash columns. After re-capture in the final wash, DNA adsorbed to the paramagnetic beads was moved to the elution buffer output by again sliding the handle. The paramagnetic beads were released in the output well using the release magnet.
Yield Quantification
qPCR was used for relative quantification of DNA yield and as an indicator of sample integrity for downstream assays. Quantification was verified using the Qubit® 2.0 Fluorometer and dsDNA HS Assay Kit (Invitrogen; Carlsbad, CA).
Input Contaminant Exclusion from Output
Nonspecific carryover of contaminants from the input to the wash and elution wells was measured by spiking tartrazine(Sigma-Aldrich, St. Louis, MO) into the sample input. A CLARIOstar® microplate reader(BMG LABTECH; Ortenberg, Germany) was used to generate standard curves and quantitate the fraction of contaminant tartrazine from the input transferred to subsequent wells.
Maintenance of Sample Integrity
The potential for cross-contamination was tested by loading an EXTRACTMAN® microplate with MCF7 and cornhusk lysate, alternating mammalian and plant samples in the four rows A-D in the EXTRACTMAN® microplate. After DNA isolation all samples were tested for mammalian and plant DNA by PCR using prime/probe sets for GAPDH and corn alcohol dehydrogenase(ADH) respectively(Applied Biosystems; Carlsbad, CA).
RESULT
EXTRACTMAN® Achieves DNA Yield Equivalent to Conventional Protocols
Yield was used as the first metric of EXTRACTMAN® performance. For greatest utility, a protocol executed with EXTRACTMAN® should yield equivalent or increased quantities of purified DNA as compared to existing tube-based, paramagnetic bead-based protocols. To demonstrate the flexibility of EXTRACTMAN® in accommodating a range of commercial paramagnetic bead-based DNA isolation kits, we compared kits from three different companies. In each case, isolation of human DNA from lysate was performed with EXTRACTMAN® and with a standard magnetic tube rack. DNA quantification for isolation outputs by Qubit assay demonstrated equivalent or improved yield for EXTRACTMAN® as compared to tube-based protocols(Figure 5).
The three kits also performed comparably to each other using the two techniques. Further, qPCR analysis was in agreement with the Qubit data(Inversely proportional) and demonstrated the suitability of all isolated DNA samples for downstream analysis (Figure 5).
More than 99% of Starting Contaminants Excluded from EXTRACTMAN® Output
Tartrazine, a food dye with a broad linear range of absorbance at a wavelength of 450nm, was used as a reporter contaminant to assess and quantify the extent to which nonspecific carryover might contribute to contamination of the purified sample. Absorbance values for the elution well indicate that more than 99% of the input tartrazine was eliminated.
Figure 5. Qubit(Top) and qPCR yield(Bottom) quantification of DNA isolated from 10,000 MCF7-GFP cells using EXTRACTMAN®(Blue) vs. tube-based (green) protocols for three kits.
Figure 6. Quantification of carryover of input contaminants to output with the EXTRACTMAN®. Test for cross contamination of samples processed simultaneously in the same EXTRACTMAN® microplate.
Left: more than 99% of contaminating tartrazine dye is removed during the process. Right: no cross-contamination was seen between wells, as assayed by species-specific qPCR.
Sample Integrity is Maintained Throughout
EXTRACTMAN® Purification Principles of surface tension and surface hydrophobicity incorporated into the design of EXTRACTMAN® eliminate the potential for cross contamination of samples.
The maintenance of sample integrity was tested using highly sensitive PCR quantification of species-specific genes in the outputs from alternating plant and mammalian lysates processed in parallel on the same EXTRACTMAN® microplate. There was no evidence of sample cross-contamination as the human GAPDH gene was detected only in human sample lanes and detection of corn alcohol dehydrogenase(ADH) was limited to lanes containing cornhusk lysate.
SUMMARY
•EXTRACTMAN® utilizes the simple and robust ESP™ technique and its operation is rapid, efficient, and straightforward-just slide the handle to bind, wash, and elute.
•The bead capture strips enable high yield purification of multiple samples in parallel with no cross-contamination, loss in yield, or compromise in sample purity and integrity.
•EXTRACTMAN® technology is flexible, and is compatible with a variety of magnetic-based kits for isolation of DNA, proteins, and other analytes.
'EXTRACTMAN® : Rapid, Reproducible DNA Extraction with High Yield'에 대한 궁금한 내용은 본 원고자료를 제공한 한국분석기기(주)를 통하여 확인할 수 있다.
Reference(참고문헌) :
1. Berry SM, Chin EN, Jackson SS, Strotman LN, Goel M, Thompson NE, Alexander CM, Miyamoto S, Burgess RR, Beebe DJ. Weak protein-protein interactions revealed by immiscible filtration assisted by surface tension. Anal. Biochem. 15:447:133-40.
2. Casavant BP, Guckenberger DJ, Beebe DJ, Berry SM. Efficient sample preparation from complex biological samples using a sliding lid for immobilized droplet extractions. Anal. Chem. 86: 6355-62.
3. Rahman MM, Elaissari A. Nucleic acid sample preparation for in vitro molecular diagnosis: from conventional techniques to biotechnology. Drug Disc. Today. 17: 1199-207.
Gilson Application note 1006 (pp1-5)
Trademarks
All product and company names are trademarks™ or registered® trademarks of their respective holders. Use of the trademark(s) in this document does not imply any affiliation with or endorsements by the trademark holder(s).
Agencourt® and DNAdvance™(Beckman Coulter)
Exclusion-based Sample Preparation(ESP™) (Salus Discovery)
Dynabeads® and DNA DIRECT™(Life Technologies)
Magnesil®(Promega)
Qubit®(Invitrogen)
CLARIOstar®(BMG LABTECH)
Model Name(모델명): EXTRACTMAN®
The Person in Charge(담당자): Hyesook An
Maker(제조사): Gilson
Country of Origin(원산지): USA
이메일: kaisco1@kaisco.co.kr
Data Services(자료제공): KAISCO.
<이 기사는 사이언스21 매거진 2021년 1월호에 게재 되었습니다.>
 Figure 1. EXTRACTMAN® includes a base and handle and uses disposable microplates and bead capture strips.The technology makes use of paramagnetic beads, also referred to as paramagnetic particles(PMP), with surfaces that bind specific analytes. Selective binding enables exclusion-based sample preparation(ESP™), a class of simple and fast purification techniques in which analyte bound to paramagnetic beads is pulled through a liquid interface into a second phase(air or liquid). Because ESP uses surface tension to gently and efficiently exclude unbound material from being transferred to the next phase, even weakly-bound species can be isolated.
Figure 1. EXTRACTMAN® includes a base and handle and uses disposable microplates and bead capture strips.The technology makes use of paramagnetic beads, also referred to as paramagnetic particles(PMP), with surfaces that bind specific analytes. Selective binding enables exclusion-based sample preparation(ESP™), a class of simple and fast purification techniques in which analyte bound to paramagnetic beads is pulled through a liquid interface into a second phase(air or liquid). Because ESP uses surface tension to gently and efficiently exclude unbound material from being transferred to the next phase, even weakly-bound species can be isolated.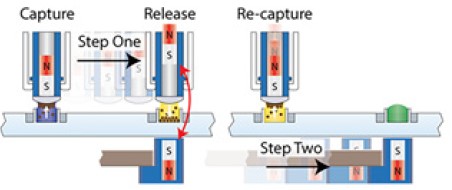 Figure 2. EXTRACTMAN® is operated by repetition of two basic user movements after paramagnetic beads are first captured: 1) slide handle to release the paramagnetic beads and 2) slide release magnet to re-capture paramagnetic beads.Release is achieved when the capture magnets in the handle and release magnets in the base are aligned over and under the same well column, repelling the capture magnets up into the handle. Re-capture is achieved by maintaining the position of the handle over the capture well and by advancing the release magnet to the next well column.
Figure 2. EXTRACTMAN® is operated by repetition of two basic user movements after paramagnetic beads are first captured: 1) slide handle to release the paramagnetic beads and 2) slide release magnet to re-capture paramagnetic beads.Release is achieved when the capture magnets in the handle and release magnets in the base are aligned over and under the same well column, repelling the capture magnets up into the handle. Re-capture is achieved by maintaining the position of the handle over the capture well and by advancing the release magnet to the next well column.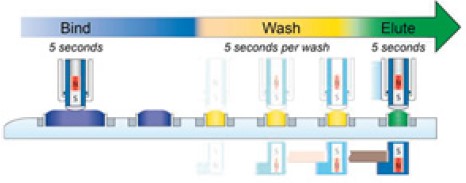 Figure 3. EXTRACTMAN® DNA isolation process using ESP™.During the bind step, DNA in the crude lysate is selectively adsorbed to the paramagnetic beads. paramagnetic bead-bound DNA is then moved through wash buffer to the elution buffer using magnetic manipulation of the paramagnetic beads. Unbound contaminants remain in the input well and nonspecific carryover is eliminated via washes. Each step in the process requires only seconds of hands-on time.EXTRACTMAN® employs novel techniques that enable ESP, offering a fundamentally new process of analyte purification(Figure 2).With EXTRACTMAN®, paramagnetic beads bound to the analyte are uniquely manipulated through the bind, wash, elute workflowusing two opposing sets of magnets: (1) free-floating capture magnets housed in the sliding handle, and (2) manually actuated release magnets, which slide below the microplate and have opposing polarity to the capture magnets. Sample preparation proceeds in two steps. The first step involves paramagnetic bead capture onto the handle’s bead capture strip followed by sliding the handle to advance the bead capture strip(and associated paramagnetic beads) from a first column of wells to a second column.The release magnet, initially positioned under the second column of wells, repels the capture magnets and attracts the paramagnetic beads from bead capture strip into the second column of wells. In the second step, the release magnets are advanced to a successive column of wells.As a result, the capture magnets are no longer repelled by the release magnets, and fall back to the lower position to recapture the paramagnetic beads. Repeating these two steps across the microplate carries the paramagnetic beads bound to analyte from the input, through the washes, and to the output (Figure 3).“…Gilson EXTRACTMAN® was used to quickly, easily, and efficiently isolate DNA…”The process of capture-slide-release-slide allows the user to quickly perform four isolations in parallel. The simple sliding movements virtually eliminate user-to-user variability, whereas analyte recovery and purity in other existing manual techniques depend greatly on the user’s skill in the art of performing careful aspirations. Additionally, the simplicity of this ESP™ method has lent itself to straightforward automation on Gilson PIPETMAX®. Thus, the advantages of EXTRACTMAN® are easily scalable to higher throughput.This application note describes DNA isolation from cell culture lysate by three commercial kits that were simply and easily adapted for use with EXTRACTMAN®.EXTRACTMAN® achieved four DNA isolations nine times faster than the tube-based protocol (Figure 4). Translation of these commercial kits to EXTRACTMAN® resulted in improved or equivalent yield while maintaining sample purity and integrity.
Figure 3. EXTRACTMAN® DNA isolation process using ESP™.During the bind step, DNA in the crude lysate is selectively adsorbed to the paramagnetic beads. paramagnetic bead-bound DNA is then moved through wash buffer to the elution buffer using magnetic manipulation of the paramagnetic beads. Unbound contaminants remain in the input well and nonspecific carryover is eliminated via washes. Each step in the process requires only seconds of hands-on time.EXTRACTMAN® employs novel techniques that enable ESP, offering a fundamentally new process of analyte purification(Figure 2).With EXTRACTMAN®, paramagnetic beads bound to the analyte are uniquely manipulated through the bind, wash, elute workflowusing two opposing sets of magnets: (1) free-floating capture magnets housed in the sliding handle, and (2) manually actuated release magnets, which slide below the microplate and have opposing polarity to the capture magnets. Sample preparation proceeds in two steps. The first step involves paramagnetic bead capture onto the handle’s bead capture strip followed by sliding the handle to advance the bead capture strip(and associated paramagnetic beads) from a first column of wells to a second column.The release magnet, initially positioned under the second column of wells, repels the capture magnets and attracts the paramagnetic beads from bead capture strip into the second column of wells. In the second step, the release magnets are advanced to a successive column of wells.As a result, the capture magnets are no longer repelled by the release magnets, and fall back to the lower position to recapture the paramagnetic beads. Repeating these two steps across the microplate carries the paramagnetic beads bound to analyte from the input, through the washes, and to the output (Figure 3).“…Gilson EXTRACTMAN® was used to quickly, easily, and efficiently isolate DNA…”The process of capture-slide-release-slide allows the user to quickly perform four isolations in parallel. The simple sliding movements virtually eliminate user-to-user variability, whereas analyte recovery and purity in other existing manual techniques depend greatly on the user’s skill in the art of performing careful aspirations. Additionally, the simplicity of this ESP™ method has lent itself to straightforward automation on Gilson PIPETMAX®. Thus, the advantages of EXTRACTMAN® are easily scalable to higher throughput.This application note describes DNA isolation from cell culture lysate by three commercial kits that were simply and easily adapted for use with EXTRACTMAN®.EXTRACTMAN® achieved four DNA isolations nine times faster than the tube-based protocol (Figure 4). Translation of these commercial kits to EXTRACTMAN® resulted in improved or equivalent yield while maintaining sample purity and integrity.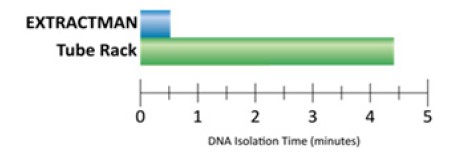 Figure 4. Time required to complete four parallel DNA isolations using either EXTRACTMAN® or standard tube-based isolations. The EXTRACTMAN® method was on average approximately nine times faster than tube-based methods.MATERIALS AND METHODSCell CultureeGFP-expressing MCF7 breast cancer cells(a generous gift of Dr. Elaine Alarid, UW-Madison, Wisconsin) were maintained in a humidified chamber at 37℃ and 5% CO₂. Culture medium consisted of DMEM with 10% FBS and 1% penicillin-streptomycin. Cells were passaged and harvested using 0.25% trypsin-EDTA.Kit AdaptationThree kits were evaluated: Agencourt® DNAdvance™(Beckman Coulter; Brea, CA); Dynabeads® DNA DIRECT™ Universal Kit(Life Technologies; Carlsbad, CA); Magnesil® Paramagnetic Particles (Promega; Madison, WI) with Buffer RLT(QIAGEN; Germantown, MD). Tube‑based DNA isolations were performed with a 6-Tube Magnetic Separation Rack(New England BioLabs; Ipswich, MA). Kits were used according to manufacturers’ protocols with the following modifications: DNA was isolated from 104 cells for all kits, final volume for elution was 100μL for all kits, cell culture samples were used in place of tissue, lysis/digestion was shortened to 1 hour for AGENCOURT DNAdvance kit.For EXTRACTMAN® isolations the DNAdvance and Dynabeads DNA DIRECT Universal Kit were further modified: sample lysate input volume was 420μL for all kits(maintaining 104 cells/isolation and paramagnetic bead number was the same as the tube-based equivalent), all wash and elution volumes were 100μL.EXTRACTMAN® Device OperationParamagnetic bead-bound DNA was captured in the input and transferred to the wash wells by sliding the handle with the paramagnetic beads held in place on the bead capture strip by the capture magnets. The paramagnetic beads were released into the wash wells and re-captured by moving the release magnet under and away from the current wash well respectively. This wash sequence was repeated for the appropriate number of wash columns. After re-capture in the final wash, DNA adsorbed to the paramagnetic beads was moved to the elution buffer output by again sliding the handle. The paramagnetic beads were released in the output well using the release magnet.Yield QuantificationqPCR was used for relative quantification of DNA yield and as an indicator of sample integrity for downstream assays. Quantification was verified using the Qubit® 2.0 Fluorometer and dsDNA HS Assay Kit (Invitrogen; Carlsbad, CA).Input Contaminant Exclusion from OutputNonspecific carryover of contaminants from the input to the wash and elution wells was measured by spiking tartrazine(Sigma-Aldrich, St. Louis, MO) into the sample input. A CLARIOstar® microplate reader(BMG LABTECH; Ortenberg, Germany) was used to generate standard curves and quantitate the fraction of contaminant tartrazine from the input transferred to subsequent wells.Maintenance of Sample IntegrityThe potential for cross-contamination was tested by loading an EXTRACTMAN® microplate with MCF7 and cornhusk lysate, alternating mammalian and plant samples in the four rows A-D in the EXTRACTMAN® microplate. After DNA isolation all samples were tested for mammalian and plant DNA by PCR using prime/probe sets for GAPDH and corn alcohol dehydrogenase(ADH) respectively(Applied Biosystems; Carlsbad, CA).RESULTEXTRACTMAN® Achieves DNA Yield Equivalent to Conventional ProtocolsYield was used as the first metric of EXTRACTMAN® performance. For greatest utility, a protocol executed with EXTRACTMAN® should yield equivalent or increased quantities of purified DNA as compared to existing tube-based, paramagnetic bead-based protocols. To demonstrate the flexibility of EXTRACTMAN® in accommodating a range of commercial paramagnetic bead-based DNA isolation kits, we compared kits from three different companies. In each case, isolation of human DNA from lysate was performed with EXTRACTMAN® and with a standard magnetic tube rack. DNA quantification for isolation outputs by Qubit assay demonstrated equivalent or improved yield for EXTRACTMAN® as compared to tube-based protocols(Figure 5).The three kits also performed comparably to each other using the two techniques. Further, qPCR analysis was in agreement with the Qubit data(Inversely proportional) and demonstrated the suitability of all isolated DNA samples for downstream analysis (Figure 5).More than 99% of Starting Contaminants Excluded from EXTRACTMAN® OutputTartrazine, a food dye with a broad linear range of absorbance at a wavelength of 450nm, was used as a reporter contaminant to assess and quantify the extent to which nonspecific carryover might contribute to contamination of the purified sample. Absorbance values for the elution well indicate that more than 99% of the input tartrazine was eliminated.
Figure 4. Time required to complete four parallel DNA isolations using either EXTRACTMAN® or standard tube-based isolations. The EXTRACTMAN® method was on average approximately nine times faster than tube-based methods.MATERIALS AND METHODSCell CultureeGFP-expressing MCF7 breast cancer cells(a generous gift of Dr. Elaine Alarid, UW-Madison, Wisconsin) were maintained in a humidified chamber at 37℃ and 5% CO₂. Culture medium consisted of DMEM with 10% FBS and 1% penicillin-streptomycin. Cells were passaged and harvested using 0.25% trypsin-EDTA.Kit AdaptationThree kits were evaluated: Agencourt® DNAdvance™(Beckman Coulter; Brea, CA); Dynabeads® DNA DIRECT™ Universal Kit(Life Technologies; Carlsbad, CA); Magnesil® Paramagnetic Particles (Promega; Madison, WI) with Buffer RLT(QIAGEN; Germantown, MD). Tube‑based DNA isolations were performed with a 6-Tube Magnetic Separation Rack(New England BioLabs; Ipswich, MA). Kits were used according to manufacturers’ protocols with the following modifications: DNA was isolated from 104 cells for all kits, final volume for elution was 100μL for all kits, cell culture samples were used in place of tissue, lysis/digestion was shortened to 1 hour for AGENCOURT DNAdvance kit.For EXTRACTMAN® isolations the DNAdvance and Dynabeads DNA DIRECT Universal Kit were further modified: sample lysate input volume was 420μL for all kits(maintaining 104 cells/isolation and paramagnetic bead number was the same as the tube-based equivalent), all wash and elution volumes were 100μL.EXTRACTMAN® Device OperationParamagnetic bead-bound DNA was captured in the input and transferred to the wash wells by sliding the handle with the paramagnetic beads held in place on the bead capture strip by the capture magnets. The paramagnetic beads were released into the wash wells and re-captured by moving the release magnet under and away from the current wash well respectively. This wash sequence was repeated for the appropriate number of wash columns. After re-capture in the final wash, DNA adsorbed to the paramagnetic beads was moved to the elution buffer output by again sliding the handle. The paramagnetic beads were released in the output well using the release magnet.Yield QuantificationqPCR was used for relative quantification of DNA yield and as an indicator of sample integrity for downstream assays. Quantification was verified using the Qubit® 2.0 Fluorometer and dsDNA HS Assay Kit (Invitrogen; Carlsbad, CA).Input Contaminant Exclusion from OutputNonspecific carryover of contaminants from the input to the wash and elution wells was measured by spiking tartrazine(Sigma-Aldrich, St. Louis, MO) into the sample input. A CLARIOstar® microplate reader(BMG LABTECH; Ortenberg, Germany) was used to generate standard curves and quantitate the fraction of contaminant tartrazine from the input transferred to subsequent wells.Maintenance of Sample IntegrityThe potential for cross-contamination was tested by loading an EXTRACTMAN® microplate with MCF7 and cornhusk lysate, alternating mammalian and plant samples in the four rows A-D in the EXTRACTMAN® microplate. After DNA isolation all samples were tested for mammalian and plant DNA by PCR using prime/probe sets for GAPDH and corn alcohol dehydrogenase(ADH) respectively(Applied Biosystems; Carlsbad, CA).RESULTEXTRACTMAN® Achieves DNA Yield Equivalent to Conventional ProtocolsYield was used as the first metric of EXTRACTMAN® performance. For greatest utility, a protocol executed with EXTRACTMAN® should yield equivalent or increased quantities of purified DNA as compared to existing tube-based, paramagnetic bead-based protocols. To demonstrate the flexibility of EXTRACTMAN® in accommodating a range of commercial paramagnetic bead-based DNA isolation kits, we compared kits from three different companies. In each case, isolation of human DNA from lysate was performed with EXTRACTMAN® and with a standard magnetic tube rack. DNA quantification for isolation outputs by Qubit assay demonstrated equivalent or improved yield for EXTRACTMAN® as compared to tube-based protocols(Figure 5).The three kits also performed comparably to each other using the two techniques. Further, qPCR analysis was in agreement with the Qubit data(Inversely proportional) and demonstrated the suitability of all isolated DNA samples for downstream analysis (Figure 5).More than 99% of Starting Contaminants Excluded from EXTRACTMAN® OutputTartrazine, a food dye with a broad linear range of absorbance at a wavelength of 450nm, was used as a reporter contaminant to assess and quantify the extent to which nonspecific carryover might contribute to contamination of the purified sample. Absorbance values for the elution well indicate that more than 99% of the input tartrazine was eliminated.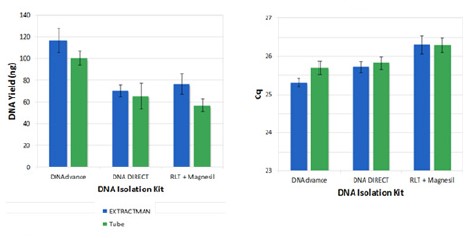 Figure 5. Qubit(Top) and qPCR yield(Bottom) quantification of DNA isolated from 10,000 MCF7-GFP cells using EXTRACTMAN®(Blue) vs. tube-based (green) protocols for three kits.
Figure 5. Qubit(Top) and qPCR yield(Bottom) quantification of DNA isolated from 10,000 MCF7-GFP cells using EXTRACTMAN®(Blue) vs. tube-based (green) protocols for three kits.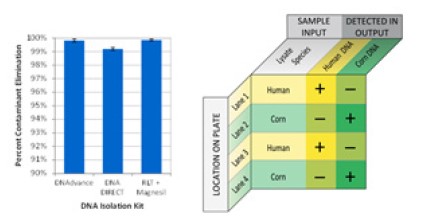 Figure 6. Quantification of carryover of input contaminants to output with the EXTRACTMAN®. Test for cross contamination of samples processed simultaneously in the same EXTRACTMAN® microplate.Left: more than 99% of contaminating tartrazine dye is removed during the process. Right: no cross-contamination was seen between wells, as assayed by species-specific qPCR.Sample Integrity is Maintained ThroughoutEXTRACTMAN® Purification Principles of surface tension and surface hydrophobicity incorporated into the design of EXTRACTMAN® eliminate the potential for cross contamination of samples.The maintenance of sample integrity was tested using highly sensitive PCR quantification of species-specific genes in the outputs from alternating plant and mammalian lysates processed in parallel on the same EXTRACTMAN® microplate. There was no evidence of sample cross-contamination as the human GAPDH gene was detected only in human sample lanes and detection of corn alcohol dehydrogenase(ADH) was limited to lanes containing cornhusk lysate.SUMMARY•EXTRACTMAN® utilizes the simple and robust ESP™ technique and its operation is rapid, efficient, and straightforward-just slide the handle to bind, wash, and elute.•The bead capture strips enable high yield purification of multiple samples in parallel with no cross-contamination, loss in yield, or compromise in sample purity and integrity.•EXTRACTMAN® technology is flexible, and is compatible with a variety of magnetic-based kits for isolation of DNA, proteins, and other analytes.'EXTRACTMAN® : Rapid, Reproducible DNA Extraction with High Yield'에 대한 궁금한 내용은 본 원고자료를 제공한 한국분석기기(주)를 통하여 확인할 수 있다.Reference(참고문헌) :1. Berry SM, Chin EN, Jackson SS, Strotman LN, Goel M, Thompson NE, Alexander CM, Miyamoto S, Burgess RR, Beebe DJ. Weak protein-protein interactions revealed by immiscible filtration assisted by surface tension. Anal. Biochem. 15:447:133-40.2. Casavant BP, Guckenberger DJ, Beebe DJ, Berry SM. Efficient sample preparation from complex biological samples using a sliding lid for immobilized droplet extractions. Anal. Chem. 86: 6355-62.3. Rahman MM, Elaissari A. Nucleic acid sample preparation for in vitro molecular diagnosis: from conventional techniques to biotechnology. Drug Disc. Today. 17: 1199-207.Gilson Application note 1006 (pp1-5)TrademarksAll product and company names are trademarks™ or registered® trademarks of their respective holders. Use of the trademark(s) in this document does not imply any affiliation with or endorsements by the trademark holder(s).Agencourt® and DNAdvance™(Beckman Coulter)Exclusion-based Sample Preparation(ESP™) (Salus Discovery)Dynabeads® and DNA DIRECT™(Life Technologies)Magnesil®(Promega)Qubit®(Invitrogen)CLARIOstar®(BMG LABTECH)Model Name(모델명): EXTRACTMAN®The Person in Charge(담당자): Hyesook AnMaker(제조사): GilsonCountry of Origin(원산지): USA이메일: kaisco1@kaisco.co.krData Services(자료제공): KAISCO.<이 기사는 사이언스21 매거진 2021년 1월호에 게재 되었습니다.>
Figure 6. Quantification of carryover of input contaminants to output with the EXTRACTMAN®. Test for cross contamination of samples processed simultaneously in the same EXTRACTMAN® microplate.Left: more than 99% of contaminating tartrazine dye is removed during the process. Right: no cross-contamination was seen between wells, as assayed by species-specific qPCR.Sample Integrity is Maintained ThroughoutEXTRACTMAN® Purification Principles of surface tension and surface hydrophobicity incorporated into the design of EXTRACTMAN® eliminate the potential for cross contamination of samples.The maintenance of sample integrity was tested using highly sensitive PCR quantification of species-specific genes in the outputs from alternating plant and mammalian lysates processed in parallel on the same EXTRACTMAN® microplate. There was no evidence of sample cross-contamination as the human GAPDH gene was detected only in human sample lanes and detection of corn alcohol dehydrogenase(ADH) was limited to lanes containing cornhusk lysate.SUMMARY•EXTRACTMAN® utilizes the simple and robust ESP™ technique and its operation is rapid, efficient, and straightforward-just slide the handle to bind, wash, and elute.•The bead capture strips enable high yield purification of multiple samples in parallel with no cross-contamination, loss in yield, or compromise in sample purity and integrity.•EXTRACTMAN® technology is flexible, and is compatible with a variety of magnetic-based kits for isolation of DNA, proteins, and other analytes.'EXTRACTMAN® : Rapid, Reproducible DNA Extraction with High Yield'에 대한 궁금한 내용은 본 원고자료를 제공한 한국분석기기(주)를 통하여 확인할 수 있다.Reference(참고문헌) :1. Berry SM, Chin EN, Jackson SS, Strotman LN, Goel M, Thompson NE, Alexander CM, Miyamoto S, Burgess RR, Beebe DJ. Weak protein-protein interactions revealed by immiscible filtration assisted by surface tension. Anal. Biochem. 15:447:133-40.2. Casavant BP, Guckenberger DJ, Beebe DJ, Berry SM. Efficient sample preparation from complex biological samples using a sliding lid for immobilized droplet extractions. Anal. Chem. 86: 6355-62.3. Rahman MM, Elaissari A. Nucleic acid sample preparation for in vitro molecular diagnosis: from conventional techniques to biotechnology. Drug Disc. Today. 17: 1199-207.Gilson Application note 1006 (pp1-5)TrademarksAll product and company names are trademarks™ or registered® trademarks of their respective holders. Use of the trademark(s) in this document does not imply any affiliation with or endorsements by the trademark holder(s).Agencourt® and DNAdvance™(Beckman Coulter)Exclusion-based Sample Preparation(ESP™) (Salus Discovery)Dynabeads® and DNA DIRECT™(Life Technologies)Magnesil®(Promega)Qubit®(Invitrogen)CLARIOstar®(BMG LABTECH)Model Name(모델명): EXTRACTMAN®The Person in Charge(담당자): Hyesook AnMaker(제조사): GilsonCountry of Origin(원산지): USA이메일: kaisco1@kaisco.co.krData Services(자료제공): KAISCO.<이 기사는 사이언스21 매거진 2021년 1월호에 게재 되었습니다.>